A small device that patients can swallow in any outpatient setting without being under sedation should allow for earlier detection of Barrett’s esophagus (BE) and thereby prevent the development of esophageal adenocarcinoma (EAC), which can arise from these premalignant lesions.
The device, which is about the size of a vitamin pill, could be used for screening instead of the more invasive endoscopy, say the researchers. It could be used as an easy, inexpensive “one-time” test that physicians can use to screen all patients older than 50 years, the researchers suggest.
The research was published online January 17 in Science Translational Medicine.
“Our current understanding is that EACs virtually all arise from BE,” senior author Sanford Markowitz, MD, PhD, Ingalls professor of cancer genetics, Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, Ohio, told Medscape Medical News.
We should be able to prevent the greater majority of EACs from ever developing.
“If we could identify everyone with BE and resect or ablate the BE when it shows dysplasia, we should be able to prevent the greater majority of EACs from ever developing,” he added.
The incidence of EAC has “steadily increased” in the recent past, and esophageal cancer is still associated with a poor prognosis, the authors note.
Patients at highest risk for BE are white men older than 50 who have persistent heartburn.
Typically, to confirm the diagnosis for these high-risk patients, physicians perform endoscopy and biopsy of the target lesion.
“If the biopsy shows changes towards becoming frank cancer, then the Barrett’s can be removed through the endoscopy by a process called endomucosal resection,” Dr Markowitz explained. Alternatively, lesions can be burned away with radiofrequency ablation or frozen off through cryoablation.
The new device, which is less invasive than endoscopy, could be used to screen all individuals older than 50, instead of only patients at high risk, the researchers suggest.
The team is actively seeking partners who can help them with the commercial development and distribution of their assay. National, multicenter trials are expected to be launched sometime this year.
Developing the Device
In developing the new device, the team first performed a genome-wide screening exercise to identify DNA methylation biomarkers that would signify the presence of BE through a molecular assay.
From this screening, they identified two biomarkers, CCNA1 and VIM DNA methylation. They tested the validity as biomarkers for BE in cytology brushings taken from the distal esophagus of 173 patients who either had BE or who did not.
Using bisulfate sequencing analysis, the researchers showed that molecular cytology assays of these brushings could detect both BE and EAC with a sensitivity and a specificity greater than 90%.
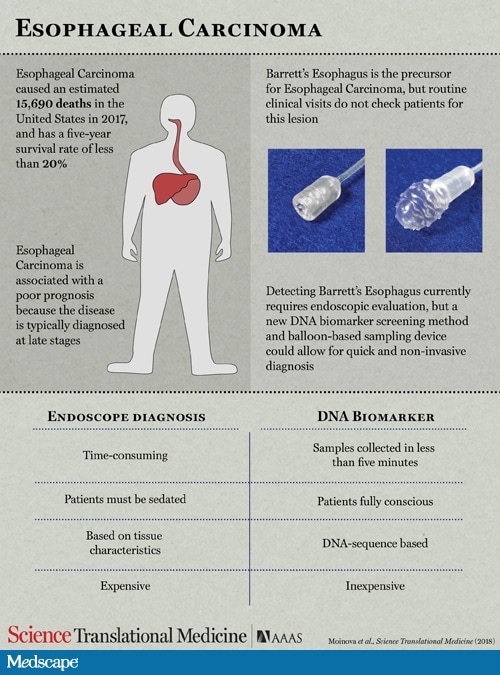
They then developed a novel, nonendoscopic screening device ― a swallowable, encapsulated balloon designed to sample the distal esophagus in less than 5 min.
The device is a swallowed 16- × 9-mm capsule attached to a 2.16-mm silicone catheter. After it is swallowed and delivered to the stomach, the balloon is inflated. It is then gently withdrawn back through the distal esophagus, where it gathers DNA from the epithelial surface.
“The balloon is then deflated and inverted back into the capsule, thus protecting the acquired biosample from further dilution or contamination in the proximal esophagus and oropharynx,” the authors explain.
The DNA is subsequently extracted from inside the device, and molecular analysis is conducted, they explain.
Sampling Device Tested
The device was tested in 156 individuals who were scheduled to undergo a esophagogastroduodenoscopy. Most of the participants were male (71%) and white (83%); the average age of the patients was 64.1 years.
Of the 156 patients, 28 (18%) were not able to swallow the device, and 128 (82%) were able to do so. Among those who swalloed the device, there were few to no reports of anxiety, pain, or choking. Only low to intermediate levels of gagging were reported.
“On average, the balloon reached the stomach in 3.3 minutes…with excellent tolerance in 72% of cases,” the authors report.
Importantly, of the 128 balloons subjected to molecular analysis following the swallowing trial, adequate amounts of DNA were found in 91% of the samples.
“In balloon samples from 86 individuals, tests of CCNA1 plus VIM DNA methylation detected BE metaplasia with 90.3% sensitivity and 91.7% specificity,” the investigators report — a yield that was nearly identical to that found when cytology brushing samples were analyzed, they add.
“This study suggests that the combination of a balloon-based sampling device with bisulfate sequencing of the VIM and CCNA1 loci provides a highly sensitive and specific yet minimally invasive screening procedure that could be clinically useful for detection and screening of BE,” investigators conclude.
Several of the investigators, including Dr Markowitz, have been awarded patents on the use of methylated vimentin for the detection of BE and other gastrointestinal cancers and have a patent pending on a balloon-based device for nonendoscopic sampling of the esophagus. Some investigators also have patents pending on methylated CCNA1. Patent rights have been assigned to Case Western Reserve University and are managed under institutional conflict of interest policies. Dr Markowitz has consulting relationships with Rodeo Therapeutics, Janssen Pharmaceuticals, and GlaxoSmithKline.Other investigators also report financial relationships with industry, which are listed in the original article.
Sci Transl Med. Published online January 17, 2018. Full text
For more from Medscape Oncology, follow us on Twitter: @MedscapeOnc
Tidak ada komentar:
Posting Komentar