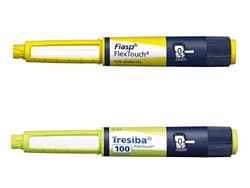
The color red will be added to the yellow label for the injection pen of faster-acting insulin aspart (Fiasp, Novo Nordisk) in order to avoid mix-ups with the light-green label of insulin degludec (Tresiba, Novo Nordisk) injection pen.
Courtesy of Novo Nordisk
The move follows reports of diabetes patients mistakenly injecting faster aspart instead of degludec, or the other way around, given that the light green and yellow may be difficult to distinguish from each other in low lighting.

Courtesy of Novo Nordisk
The announcement of the label color change was made March 23 by the European Medicines Agency (EMA), following a March 9th positive opinion “related to the update to the colour design, adding contrasting red assets to the label design, package and selected plastic components of the Fiasp® vial, Penfill® cartridge and FlexTouch® pen, as well as safety information to be distributed to pharmacies and dispensing clinics,” according to a statement from Novo Nordisk provided to Medscape Medical News.
The EMA provides the following advice to healthcare professionals:
-
When dispensing or prescribing Fiasp, check whether the patient also uses Tresiba.
-
If so, remind the patient to be careful not to mix up these two medicines because of the risk for hypo- or hyperglycemia.
-
Advise patients to check the name of the insulin before each injection and to take extra care if preparing injections in poor light.
-
Adverse reactions relating to Fiasp or Tresiba, including medication errors, should be reported to Novo Nordisk or to the appropriate local authorities.
Novo Nordisk is establishing country-specific dialogues with local health authorities in Europe regarding implementation of these changes, company representative Adam Pittard told Medscape.
He said that the plan is to implement the label change worldwide, “but all updates depend on regulatory approvals granted by the different authorities.
“As of now, we can confirm that the Fiasp® presentations will be updated in all European Union state members, including Norway and Iceland. This is the same for approvals in Canada and Switzerland, where we have conducted similar regulatory processes.”
In the United States, the company said, “It is our plan to update the selected plastic components of the Fiasp pen in the near future…and we will collaborate with the FDA [Food and Drug Administration] on the implementation.”
For more diabetes and endocrinology news, follow us on Twitter and on Facebook
Tidak ada komentar:
Posting Komentar