Ferumoxytol (Feraheme, AMAG Pharmaceuticals), indicated in the treatment of iron deficiency in chronic kidney disease, is gaining interest as a contrast agent for vascular MRI that not only is safer than conventional gadolinium-based agents but allows imaging to be completed in a fraction of the time — an important feature for people who struggle with the discomfort of a lengthy scan.
In a new report, radiologists with the David Geffen School of Medicine at the University of California Los Angeles (UCLA) described using ferumoxytol as a contrast agent in vascular imaging of the thorax, abdomen, and pelvis in seven patients who had renal failure as well as claustrophobia.
While MR angiography (MRA) using a gadolinium contrast agent can take between 30 minutes and an hour, the patients, who had all reported having claustrophobia and a reluctance to undergo the discomfort of the lengthy MR examination, were scanned at an average time of just 6.27 minutes (range, 4.16 to 10.10 minutes), with a tune-up time of less than 1 minute.
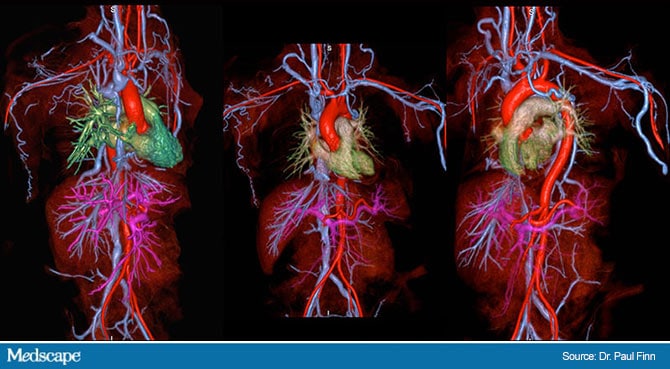
The scans were all described as high diagnostic quality, providing full visualization of arterial and venous anatomy extending from the neck to the thighs.
“This method could enable claustrophobic patients to receive lifesaving diagnoses and procedures,” said first author, Puja Shahrouki, a research fellow with the David Geffen School of Medicine at UCLA, in a press statement.
“For appropriate types of studies, it could also shorten waiting lists and improve the cost-benefit ratio for hospitals.”
The findings were presented at CMR 2018, a joint meeting of EuroCMR — the annual European Association of Cardiovascular Imaging meeting on cardiovascular magnetic resonance — and the Society for Cardiovascular Magnetic Resonance.
Senior author, J Paul Finn, MD, also from the David Geffen School of Medicine at UCLA, added that while approximately 2% of patients undergoing MRI have claustrophobia, that probably falls far short of the actual rate.
“We suspect the number would be higher if the patients with claustrophobia that refuse to be referred to MRIs are accounted for,” he told Medscape Medical News.
Computed tomography angiography (CTA), the more common approach to vascular imaging, can allow for imaging in as little as a second. However, that method requires a timed bolus of intravenous (IV) iodinated contrast agent that, in addition to possibly presenting a risk for kidney damage in some patients, can involve several minutes of setup time that is not necessary with ferumoxytol, Finn explained.
“When ferumoxytol is administered before the patient goes into the MRI scanner, there is no requirement to set up for timing an IV bolus,” he said. “All that is required for vascular imaging is to do some fast localizer sequences and one or two breath-hold acquisitions.
“So, paradoxically, the procedure for ferumoxytol-enhanced MRA (FEMRA) can be even faster than modern CTA.”
Renal Concerns Driving Interest
While ferumoxytol was initially designed as a vascular MRI contrast agent, its makers instead sought and gained approval from the US Food and Drug Administration (FDA) for the agent as the first and only bolus injectable iron therapy agent for the treatment of chronic kidney disease in 2009.
Interest in ferumoxytol as an off-label contrast agent has been increasing, however, as concern has grown over a link between some gadolinium-based contrast agents and nephrogenic system fibrosis.
In addition to the quick set-up and relative safety in patients with renal failure, ferumoxytol’s intravascular half-life is an estimated 14 hours, allowing for longer imaging acquisitions and the option for repeated imaging if necessary — without the need for administration of additional contrast agent.
Conversely, CT iodinated contrast or gadolinium-based contrast agents leak from the bloodstream immediately after the first pass of the bolus and become diluted, the authors say. That means the possible need for more time and, in the case of CT, the possibility of unwanted additional exposure to radiation.
The upshot is that FEMRA may be a valuable option in selected cases. “We use it primarily in patients with chronic kidney disease and congenital heart disease, where conventional agents are either contraindicated or ineffective,” Finn said.
“We have performed approximately 500 studies in the last 5 years and about 130 last year alone, [and] demand seems to be growing rapidly.”
Another application of ferumoxytol is in imaging the venous system, which is more difficult with CTA or gadolinium MRA because of the timing issues and dilution.
“With ferumoxytol in steady state, the veins are enhanced to the same extent as the arteries and all of the blood vessels light up to the same degree,” Finn explained.
“Veins which heretofore have been virtually impossible to image now become easy to visualize. This stands to play a pivotal role in planning interventional procedures.”
In their experience with FEMRA at UCLA, Finn said image quality for arterial imaging has been “on a par with the best results for the gadolinium agents and for venous imaging is better than with CTA or MRA with the gadolinium agents,” Finn said.
“So far, we have focused mostly on chest, abdomen, and pelvis because of our patient mix, but the results will likely be generalizable.”
Hypersensitivity Reactions Prompt Black Box, Bolus Warning
An important caveat with ferumoxytol is that, in response to reports of rare but severe hypersensitivity reactions, the FDA has issued a black box warning for the agent and specifically advised against bolus injection.
With those concerns in mind, a slow infusion approach is important, Finn said.
“Since [the FDA black box issuance], slow infusion has become our routine mode of administration and, as recommended by the FDA, we monitor blood pressure and vital signs closely for at least half an hour afterwards,” Finn explained.
Several institutions are working to define the safety profile for the diagnostic applications of ferumoxytol, and UCLA has set up a multicenter Feraheme project, which so far contains data on over 1100 patient studies from five centers in the United States and Europe, with no reports of severe adverse events, he noted.
Another caveat is the cost: Ferumoxytol is approximately four to five times more expensive than gadolinium agents. A further drawback is that the agent is available only in a single therapy dose vial of 500 mg of iron in 17 mL, which is more than is required for most adult imaging studies, while small children may require only a fraction of a milliliter, Finn said.
“Unused agent must be discarded, so clearly this is not cost-efficient as currently configured,” he said.
Some key contraindications for ferumoxytol would include having a history of allergy to intravenous iron or iron overload, Finn added.
Variety of Imaging Applications
A review published in 2015 further describes more than 3 years of clinical experience using summary of FEMRA at the University of California San Francisco for applications ranging from standard clinical indications, such as pulmonary embolism, aorta, coronary, and peripheral vascular imaging, to advanced research, such as four-dimensional flow imaging and imaging to detect vascular inflammation.
First author, Michael D Hope, MD, from of the University of California San Francisco Department of Radiology, acknowledged that CTA is typically the first choice for vascular imaging but agreed that the numerous advantages of FEMRA make it ideal for some situations.
“CTA is excellent for anatomic detail and typically is used when possible,” he told theheart.org | Medscape Cardiology.
However, advantages of FEMRA over CTA include no renal failure risk, no radiation, and its ability to be coupled with dynamic evaluation of the heart and great vessels with other MRI sequences, Hope said.
“Advantages over gadolinium also include no renal risk, no brain deposition, and the longer intravascular half-life,” he added.
“The [longer half-life] enables longer, gated MRI sequences with good contrast throughout.”
Finn is on the scientific advisory panel for AMAG Pharmaceuticals, but the company did not fund the study or have any control of the researchers’ data. Finn is also on the speakers’ bureaus for and scientific advisory panels for Bayer Pharma and Bracco Diagnostics. Hope had no disclosures to report.
CMR 2018. Presented February 3, 2018.
For more news, join us on Facebook and Twitter
Tidak ada komentar:
Posting Komentar